Farmers Seek to Deploy Powerful Gene Drive
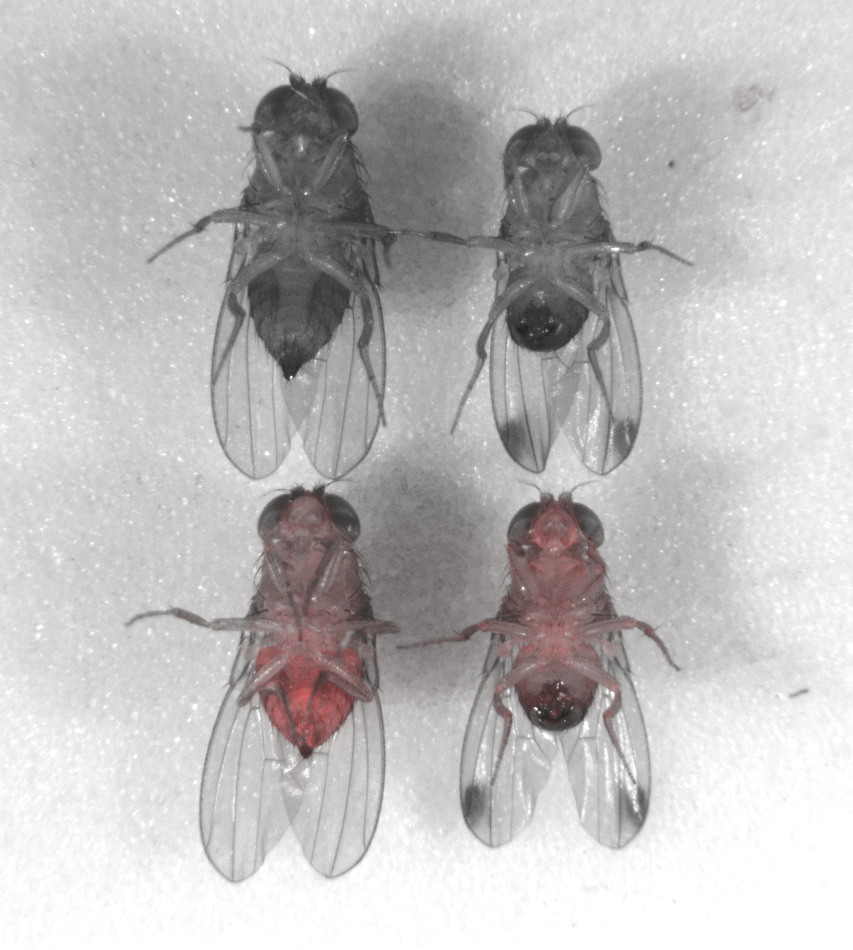
Since it first appeared in Northern California in 2008, the spotted-wing drosophila, a type of fruit fly native to Asia, has become the bane of the state’s cherry farms because of the razor-edged “ovipositor” on its tail.
Rather than lay eggs in rotting berries, as domestic flies do, the invasive species punches holes in fruit that’s still ripening, spoiling it. The costs to U.S. agriculture: about $700 million a year.
California’s cherry growers think they may have a way to get rid of the flies cheaply. To do it, they are counting on a technology developed by geneticists: a “gene drive” that can spread DNA alterations among wild flies, potentially killing them off.
Gene-drive technology is among the most widely debated—and feared—inventions of modern biology. Opponents call it a genetic “atom bomb” and want it banned. Others see the possibility of unprecedented public health interventions, like eradicating the mosquitoes that spread malaria.
Now, for the first time, commercial uses are on the table. With funding from the California Cherry Board, scientists at the University of California, Riverside, have installed a gene drive in the invasive pest, the first time the technology has been established in a commercially important species.
In addition to that effort, which remains confined to the laboratory, two spinout companies from the University of California, San Diego, are also pursuing commercial use of gene drives. One, Agragene, also intends to alter plants and insects. Its sister company, Synbal, wants to harness the technology as a speedy way of engineering lab mice and possibly pet dogs.
“It’s about having genes under precise control in whatever organism you are modifying,” says David Webb, acting CEO of both UCSD spinout companies, neither of which has raised capital.
A gene drive works via a so-called selfish gene that is able to replicate itself and get inherited by most of an animal’s offspring rather than just half, as is usual. The effect is called “super-Mendelian” inheritance.
The problem is that modifying wild animals raises complex ethical and regulatory issues. Some scientists worry that gene drives could run amok—say, if laboratory animals escape and spread changes in the wild. The Broad Institute of MIT and Harvard has even added gene drives to a list of uses of gene-editing technology it doesn't think companies should pursue.
What’s more, any use of such a powerful technology is going to be highly regulated. Such obstacles explain why most gene-drive funding has come from either philanthropies or the military. The Gates Foundation has committed more than $75 million to engineer self-destructing malaria mosquitoes, which it thinks may be needed to wipe out that disease in Africa. This year the U.S. military research agency DARPA began spending a similar amount to develop antidotes to gene drives, should they be used as a weapon.
The California Cherry Board, which represents growers, just wants to get rid of the flies. When the pests arrived a decade ago, the orchards started spraying insecticides called pyrethroids, with trade names like Mustang Maxx and Warrior.

“This is basically the strongest chemical that there is,” says Nick Matteis, an executive with the growers’ organization. The sprays kills the flies and pretty much every other insect, too, including bees. “If you didn’t have to spray, that is a huge deal,” he says.
To the cherry growers, a gene drive looks like a precision tool that could eliminate one species among thousands. In 2013, the organization started funding development of the technology, spending about $100,000 a year, or about a third of its research budget, to have Riverside professor Omar Akbari install a gene drive in that fly’s genome.
“It’s a lot of money from their perspective, but from our end, it’s only enough to pay a salary and a few experiments,” says Akbari, an expert on insect genetics and one of the participants in the DARPA program.
Even so, by July Akbari had success with the gene drive. His technology, called Medea after the Greek sorceress who murdered her children, spread to 100 percent of flies in experiments in laboratory cages, he says.
The next step it to determine what genetic cargo to attach to the selfish gene. Female flies survive the winter because their bodies make cryoprotectants. Adding a gene to block those chemicals could cause the flies to freeze. Another possibility is genetically altering the bugs’ ovipositor so that they change their behavior.
“If you got rid of that knife or dull it, instead of stabbing ripening cherries, they would lay their egg in rotting fruit, like regular flies,” says Akbari. “The flies would still exist, but they would no longer be crop pests.”
People fear that gene drives will be unstoppable once released. In fact, scientists have a wide variety of tricks to keep them under control. In Akbari’s case, his Medea system requires a large number of insects for the chain reaction to begin—at least thousands. That means a few flies hitching a ride somewhere else in a box of cherries would be unlikely to spread the drive accidentally.
The California Cherry Board says it’s now ready to finance larger-scale laboratory studies. To pay for them, and eventually seek approval to deploy a gene drive, the farmers’ group is planning to raise funds from other fruit growers to finance a “public-benefit corporation.” The company would have, as part of its charter, a requirement to keep its technical plans and finances out in the open.
“We’ll create an entity that is basically in the trust business,” says Tom Turpen, a consultant who is advising the farmers in their formation of the new company. Otherwise, he says, opponents of GMOs would likely instigate a paralyzing public debate.
Matteis, the Cherry Board executive, estimates it could be five years before a gene drive is approved and ready to deploy. He's hopeful that by then the public will support the plan. "Any insect considered remotely beneficial to the environment, you would have a much harder time," he says. "But this insect is a recent arrival. There would be less concern about disrupting the circle of life."
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.